[approx. 4 min read]
4 JULY 2022
The Australian Government has announced that self-collection Cervical Screening Tests are now available to those aged 25-74 as part of the National Cervical Screening Program, in addition to the existing option to have the screening test performed by a health care provider.¹
While this change is a welcome one for many who have previously experienced barriers to accessing the test, it is an important opportunity to dispel misconceptions around the Cervical Screening Test and highlight how vital early detection tests are in improving cancer survival rates.
What is a Cervical Screening Test?
The Cervical Screening Test is a test which checks for signs of the human papillomavirus (HPV), which is a common infection that causes most cervical cancers. Most cases of HPV clear up on their own, however some can develop into cancer.
Previous to December 2017, the Pap smear was the routine test given to women aged 18 to 69, every 2 years, to help prevent cervical cancer. However, this test has been replaced by the Cervical Screening Test, a more accurate test, and is offered every 5 years.²
National Cervical Screening Program to offer self-collection tests
The National Cervical Screening Program aims to reduce illness and death from cervical cancer in Australia through government-subsidised cervical screening tests offered to women and people with a cervix every 5 years.
You are eligible for a Cervical Screening Test if you are:
- aged between 25 and 74
- sexually active or ever have been
- a women or person with a cervix
If you have had a full or partial hysterectomy, please check with your doctor about screening.³
You can learn more about eligibility and how to access a Cervical Screening Test on the National Cervical Screening Program website here.
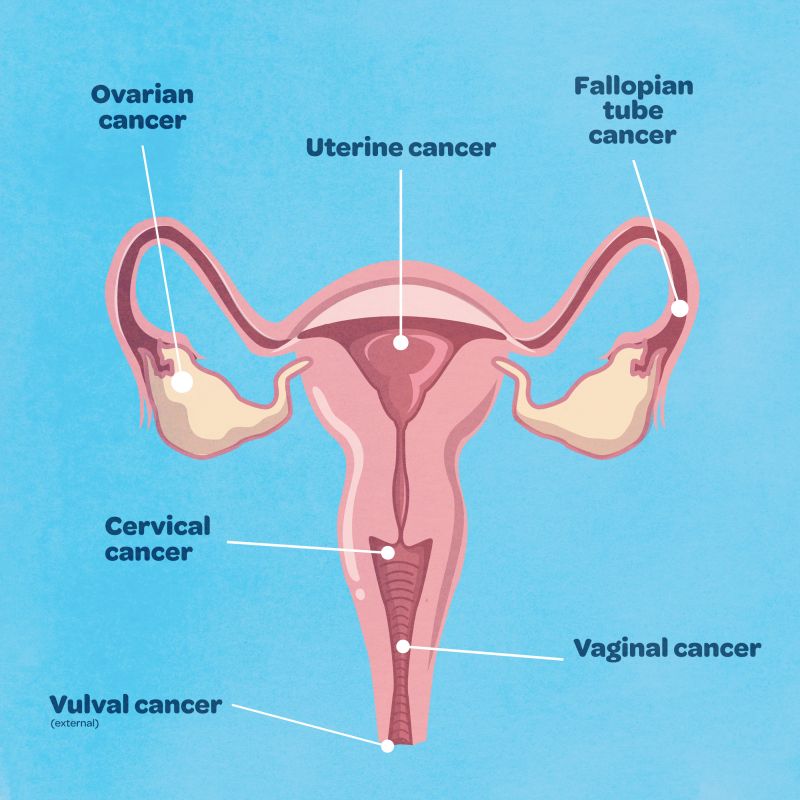
Difference between cervical cancer and ovarian cancer
Cervical cancer develops in the cervix, which is located between the vagina and the uterus.
Ovarian cancer is a tumour in one or both of the ovaries, and can also originate in the fallopian tubes.
 Source: Queensland Health
Source: Queensland Health
The incidence or number of cases of cervical cancer in Australia has significantly decreased since the introduction of the National Cervical Screening Program in 1991 and the Human Papillomavirus (HPV) vaccine program commencing in 2007. As a result, the Australian incidence and mortality rates for cervical cancer have decreased by approximately 50% since 1991, and are among the lowest in the world. It was estimated that around 913 cases of cervical cancer would be diagnosed in Australia in 2021.
The chance of surviving cervical cancer for at least 5 years is 74%.
Comparatively, the number of cases of ovarian cancer in Australia continues to grow annually, with around 1720 cases diagnosed in 2021. As there is no early detection or screening program available, the majority of cases continue to be diagnosed in the late stages, resulting in a 5-year survival rate of just 48%.
Although there are similarities between the symptoms of cervical cancer and the symptoms of ovarian cancer, the two diseases are very distinct.
Common misconceptions about the Cervical Screening Test
The Cervical Screening Test does not detect ovarian cancer. It only screens for cervical cancer.
There is a common misconception that a pap smear or cervical screening test will detect ovarian cancer - this is false. There is no early detection test for ovarian cancer. The only way to definitively diagnose ovarian cancer is through invasive surgery and tissue biopsy.
The CA125 blood test can be used to help diagnose or exclude ovarian cancer in combination with other tests and scans, however it is not accurate or specific enough to definitively diagnose ovarian cancer.
Learn more about CA125
Early detection for ovarian cancer
The development of an early detection test could lift ovarian cancer survival rates from 48% to 90% and above.
The Ovarian Cancer Research Foundation (OCRF) is funding multiple research projects examining potential biomarkers which could be utilised to detect ovarian cancer in the early stages (1a or 1b), before the disease becomes life-threatening. However, there is a need for increased funding to ensure that all promising research avenues can be provided with adequate and sustained funding.
In the absence of an early detection test, the OCRF is also focusing its grant funding on new and effective treatment methods to reduce disease recurrence and give patients facing ovarian cancer a greater quality of life.
Learn more about our research
Sources
¹ 'Pap smears' can be replaced by do-it-yourself cervical cancer tests, Department of Health and Aged Care.
² Pap Smears, Health Direct..
³ About the National Cervical Screening Program, Department of Health and Aged Care